 While “a watched pot never boils” has been proven to be incorrect, did you know there is an interesting phenomenon that makes that perception feel like reality? How about water can be at 32° F and still not be frozen? Well lets introduce you to two interesting terms; latent & sensible heat and see how they apply.
While “a watched pot never boils” has been proven to be incorrect, did you know there is an interesting phenomenon that makes that perception feel like reality? How about water can be at 32° F and still not be frozen? Well lets introduce you to two interesting terms; latent & sensible heat and see how they apply.
.
 In 1848 Elizabeth Cleghorn Stevenson released her first ever novel entitled “Mary Barton,” which is the first time the phrase “a watched pot never boils” appeared in print. The novel was set in Manchester England and detailed the hardships that the working class was enduring during the first ever industrial era depression. Just like many attribute this statement to not be impatient or anxious, that was the main point made in the story. In 1848 Elizabeth Cleghorn Stevenson released her first ever novel entitled “Mary Barton,” which is the first time the phrase “a watched pot never boils” appeared in print. The novel was set in Manchester England and detailed the hardships that the working class was enduring during the first ever industrial era depression. Just like many attribute this statement to not be impatient or anxious, that was the main point made in the story. |
Sensible Heat:
No it isn’t something that thinks or you can reason with but rather heat that is added or taken away to change the temperature of an item. In most homes we measure that in BTU’s which you might recall is the quantity of heat required to raise the temperature of one pound (16 ounces / .45 KG) of water one degree Fahrenheit. While not a perfect comparison (but very close), the heat given off by a single wooden kitchen match is one BTU.
 In the world of cooking, there are a few “levels” of boiling. Simmer is where part of the dish remains at the boiling part while the rest of the dish is not as hot (think spaghetti sauce & the occasional bubbles). In the case of the noodles you are supposed to bring the water to a rolling boil or where all the water is at 212° before placing the noodles in – an easy way to tell the difference is just stir the water, if the bubbles stop you are not at a rolling boil yet. In the world of cooking, there are a few “levels” of boiling. Simmer is where part of the dish remains at the boiling part while the rest of the dish is not as hot (think spaghetti sauce & the occasional bubbles). In the case of the noodles you are supposed to bring the water to a rolling boil or where all the water is at 212° before placing the noodles in – an easy way to tell the difference is just stir the water, if the bubbles stop you are not at a rolling boil yet. |
Getting back to our watched pot, the math is pretty simple – water boils at 212° so if the water coming out of the hot water line is at 120° we would need 92 BTU’s. So we pull out our pot, 92 matches & add 16 ounces of water. So besides some burnt fingers is the water finally boiling – nope. Well maybe it is because of the inefficiency of the delivery method, the pot, or… So another 92 matches later & all you see is a few bubbles but no rolling boil and besides wondering if your fingers will ever heal you got to wonder, what’s up with this…
 A few interesting tid-bits; the first bubbles that you see in a pot are not steam at all, it is actually dissolved air. As the water starts heating up, its ability to hold dissolved gasses decreases & these bubbles will form. Waters boiling point is affected by numerous items like elevation, and any impurities in it. Pure water at sea level will start boiling at 212°; while the higher in elevation you are the lower the temp will be. In a rush to get the noodles cooked? You might want to hold off on the salt until the water reaches a rolling boil. A few interesting tid-bits; the first bubbles that you see in a pot are not steam at all, it is actually dissolved air. As the water starts heating up, its ability to hold dissolved gasses decreases & these bubbles will form. Waters boiling point is affected by numerous items like elevation, and any impurities in it. Pure water at sea level will start boiling at 212°; while the higher in elevation you are the lower the temp will be. In a rush to get the noodles cooked? You might want to hold off on the salt until the water reaches a rolling boil. |
Latent Heat:
Latent heat (aka hidden heat) technically does not change the temperature of an item, but it is what is required to change the phase of substance from one state to another (e.g. from a solid, to liquid, to gas). One of the most well known forms of latent heat is the wator vapor found in the air. The amount of moisture in the air is one of the keys to how one might feel comfortable one day when it is 80 degrees out & yet so miserable on another day with higher humidity.
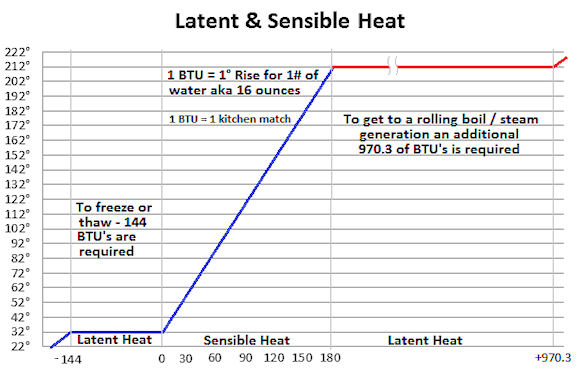
As the chart above shows, in the case of the boiling water, one would actually have to add an additional 970 BTU’s to get that rolling boil and turn it to steam. This is one reason why steam burns hurt so much more and are so bad. Now on the other end of the spectrum, in order for water to turn into ice or ice back into water an additional 144 BTU’s are needed.
 In the air conditioning world, the removal of latent heat is essentially the removal of the excess “humidity” in the air. This is also one of the major reasons that an oversized AC unit isn’t such a hot idea – it takes time to pull that humidity out & if you quickly drop the air temperature before you do that… In the air conditioning world, the removal of latent heat is essentially the removal of the excess “humidity” in the air. This is also one of the major reasons that an oversized AC unit isn’t such a hot idea – it takes time to pull that humidity out & if you quickly drop the air temperature before you do that… |
